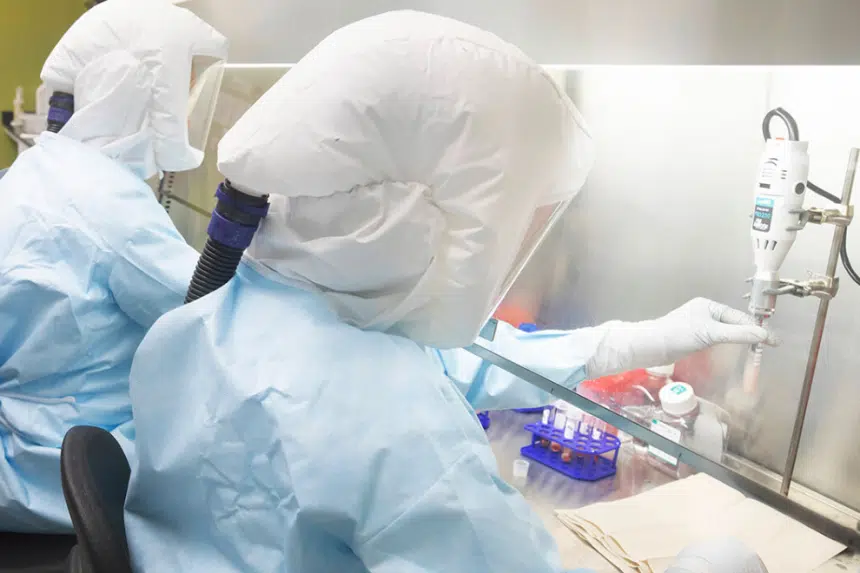
With COVID-19 now in Saskatchewan, the pressure is on for a vaccine to be found.
That work is being done by scientists around the world, including the team at the Vaccine and Infectious Disease Organization at the International Vaccine Centre (VIDO-InterVac) at the University of Saskatchewan (U of S).
The group is part of a $26.7-million federal rapid research funding initiative aimed at contributing to global efforts to contain the coronavirus outbreak.
The team at the U of S has been awarded $1 million of that money and is already beginning to see success.
“You will be happy to hear we have already immunized animals with our vaccine. So we have already made a vaccine where we are in the animal phase where we’re testing it. We’re are hoping in a few weeks from the first trial we learn whether it works or not,” said Dr. Volker Gerdts, project co-applicant and director of VIDO-InterVac.
However, Gerdts doesn’t want to get anyone’s hopes up as to what it means in real terms as the virus spreads around the globe.
“I hate to say it but it’s unfortunately a long ways to go. We are showing in an animal model that it works in principle, so that tells us this vaccine candidate might work. And then you have to further test it and demonstrate it is completely safe for use in humans,” Gerdts explained.
“We do no want to give anything that potentially causes adverse events in humans, so you do have to have safety testing, then you have to look at manufacturing issues, how to best produce it, quality control it, and then you’re ready to take it in to clinical tests in humans.
“So if everything goes really, really well, it could be ready in six to eight months to be ready to test in humans and then depending on those in 10 to 12 months there might be a candidate that’s ready to be handed out to people.”
Gerdts added the team works in collaboration with the other scientists as part of this global approach and they talk on the phone weekly to share findings, data and research.
“There’s a lot of exciting work going on right now and you can really feel it in the building (that) people are really enthusiastic and motivated,” he said.
To help improve Canada’s response and emergency preparedness to emerging threats such as this strain of coronavirus, VIDO-InterVac is building a pilot-scale vaccine manufacturing facility.
“Having a manufacturing facility would enable us to make a large amount of clinical-grade vaccine for early testing for human trials and animal models. This would help significantly reduce the response time in developing a vaccine during these emergent situations,” said Darryl Falzarano of VIDO-InterVac, who also leads one of the teams in Canada.
Through various scientific and research facilities, the federal government is funding the 47 projects across Canada.